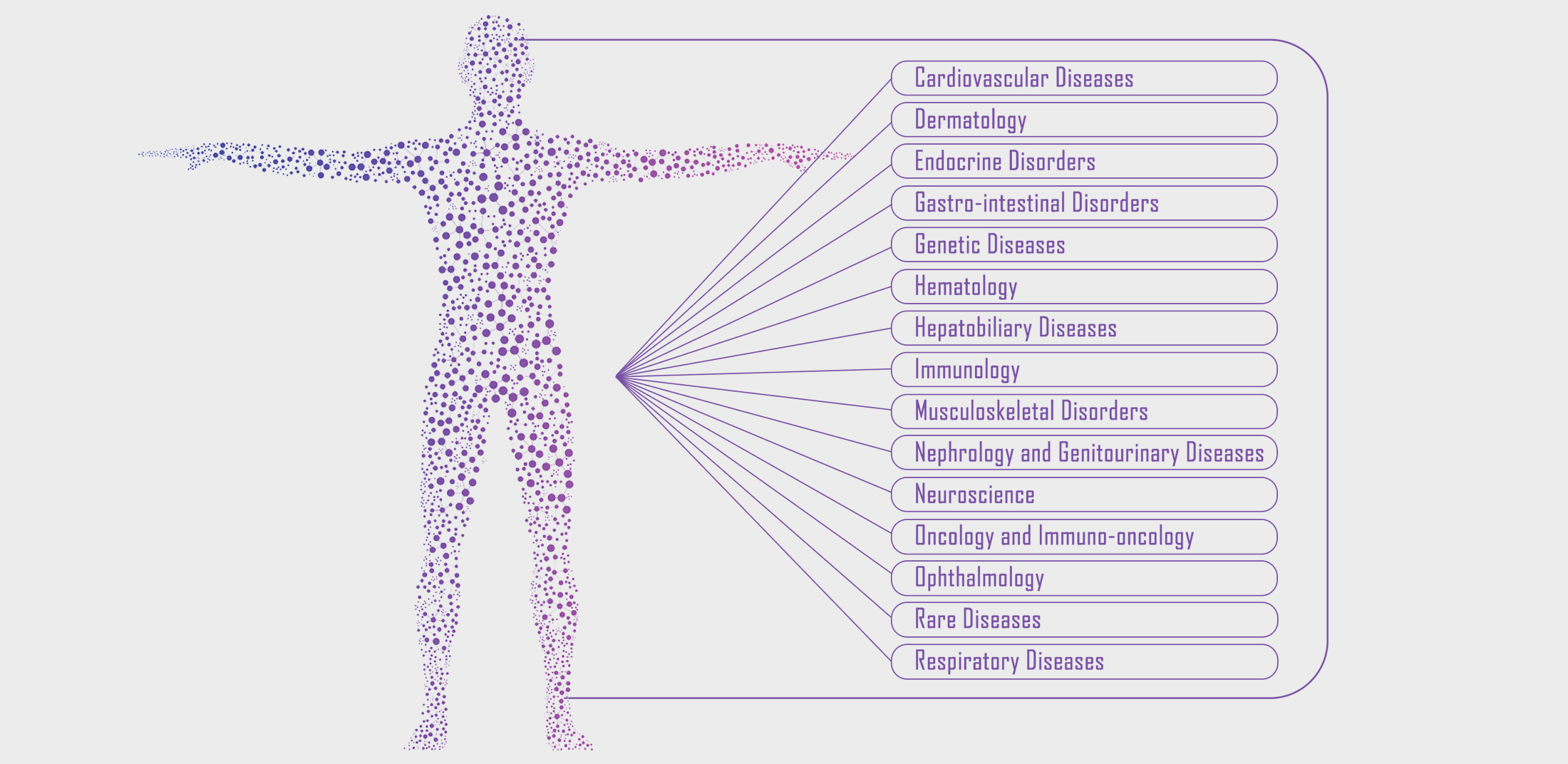
BioXcel Therapeutics, Inc. is a biopharmaceutical company developing transformative medicines in neuroscience and immuno-oncology utilizing AI, and its approaches.
Learn MoreBioXcel Therapeutics Announces Formation of OnkosXcel Therapeutics...
BioXcel Featured in Fierce Biotech The recent uptick in interest in drug repurposing, the popularity of which ebbs and flows, could give the team a tailwind..
READ MORE